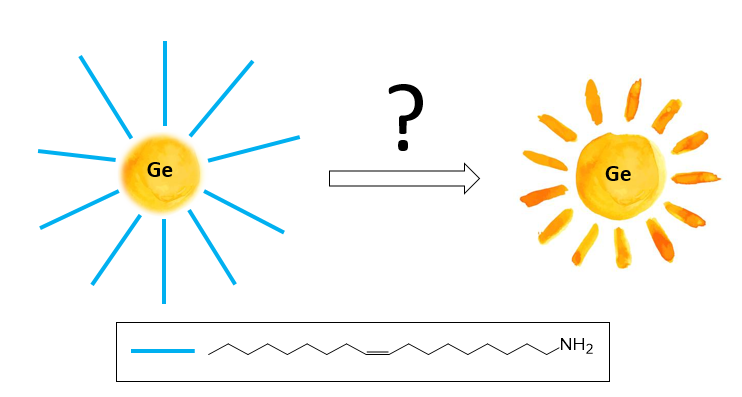
I want you to imagine the sun with wrong rays that cause a lot of problems. We cannot enjoy the sunshine. We want to do something to replace the wrong rays with the right ones. In my scientific project, the sun is germanium (Ge) nanoparticles, and the rays are a ligand. The wrong ligand is oleylamine. Oleylamine is an organic molecule containing an amino group and a long hydrocarbon chain. It would be perfect to remove oleylamine from the particles and attach another molecule. Speaking in scientific language, I am trying to do ligand exchange on germanium nanoparticles surface.
Why germanium?
Firstly, Ge is an excellent material for lithium-ion batteries (LIBs) anode. It has a higher capacity than graphitic carbon used in commercial LIBs. Secondly, Ge is a promising material for thermoelectric devices.
Why germanium nanoparticles?
However, it is still necessary to improve the exploitation of the characteristics of germanium. I will try to explain why and how. Bulk germanium electrodes crack and pulverize upon Li+ intercalation/deintercalation cycling. These negative effects could be prevented by using germanium nanomaterials instead of bulk. Nanomaterials have more space for lithium transport without pulverization due to a large surface to volume ratio. In addition to the improved mechanical properties, nanostructuring will allow enhancing thermoelectric properties. The efficiency of thermoelectric material (ZT) is determined by 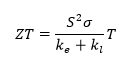
where σ is the electrical conductivity, S is the Seebeck coefficient, ke and kl are the electronic and lattice contributions to thermal conductivity, and T is the absolute temperature. Decreasing size increases power factor (S2σ) and reduces the lattice thermal conductivity kl.
Why ligand exchange?
Well, we have Ge nanoparticles which can make LIBs and thermoelectric devices much better. However, there is an important nuance that nanoscaled materials have a large surface. The quality of this surface plays a key role in the possibilities of reliable exploitation of nanoparticles. In this case, I should pay attention to the method of obtaining.

Generally, nanomaterials can be obtained by physical methods such as laser ablation and sputtering or chemical methods including colloidal synthesis. In my research project, I use the last one because production by the colloidal method does not require complex and expensive equipment and can be simple to scale. Colloidal synthesis requires a high boiling organic solvent such as long-chain primary amines which also stabilize Ge particles in the solution.
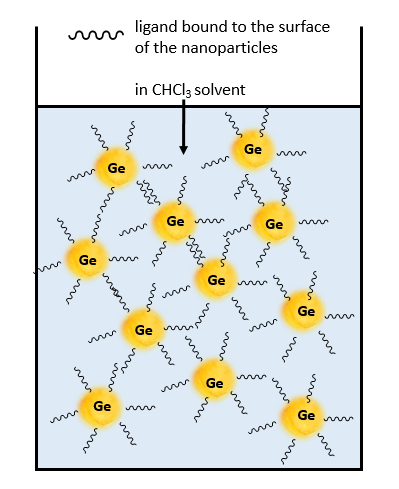
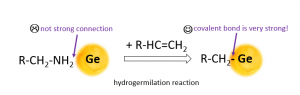
Thus, nanoparticles have long organic molecules on their surface. But long-chain primary amine is a not ideal ligand because it has no strong connection with Ge particles surface. It makes particles unstable (recollect about the sun with wrong rays). Therefore, it is reasonable to create a chemical wet route that will remove native ligand and connect with a satisfying ligand.
How am I doing this?
I am obtaining Ge particles with new ligand by hydrogermylation of colloidal germanium nanoparticles covered by oleylamine. Hydrogermylation involves the insertion of an unsaturated carbon-carbon bond into a surface-bound Ge-H bond, leading to Ge-C formation. Formation of covalent Ge-C bond provides reliable protection of the surface and lead to the creation of usable Ge nanoparticles.
It is quite difficult to carry out this reaction in practice and it is also difficult to prove that you made it. Thus, I use different methods of analysis to confirm the successful exchange of ligands among which Raman and infrared spectroscopy, nuclear magnetic resonance, photoluminescent spectroscopy.
So I told you what I’m doing and why. I wish everyone the sun with the right rays, and if it is not, you have every chance to change it. You are a scientist who can do incredible things.


Thank you for your nice post. I had a bit difficulty to understand the metaphor of sun and Germanium, maybe it would be good to start directly by explaining why Ge is so interesting as a material as you do in the second paragraph. 😉