Protein crystals are a tricky matter to study. Sometimes you can get them easily, without any effort, but sometimes to obtain one, you need to work hard and even that doesn’t bring success for a long.
Once you get the crystal, it opens the gate to a long process to determine the molecular structure. Crystals are often very delicate and can be easily destroyed by mechanical and chemical agents as well as temperature changes.
In view of these obstacles, many applications in macromolecular crystallography would benefit from the availability of a macromolecular crystal system, which exhibits outstanding diffraction properties, which is mechanically stable and radiation-hard and which can be thawed and frozen many times without concomitant loss of diffraction. Due to their large solvent content, macromolecular crystals are typically mechanically rather fragile and little suitable as the basis of such a system.
The method that gives hope to produce such a system is cross-linking using glutaraldehyde, but its use so far was only partially successful due to loss of diffraction properties of a crystal.
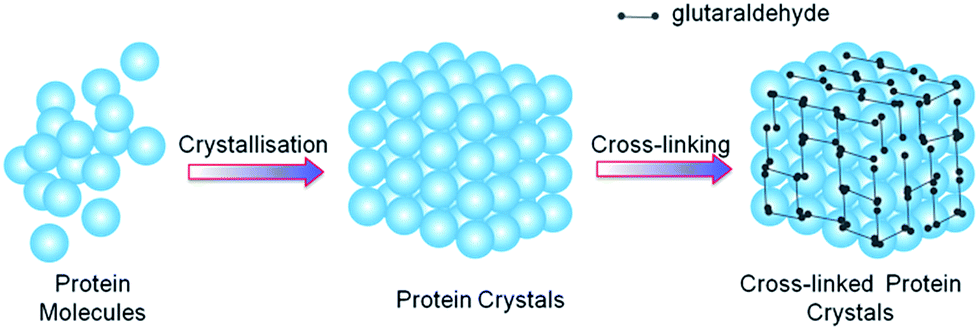
The new approach to glutaraldehyde cross-linking system assumes two steps to achieve stability of macromolecular crystals. Firstly, genetic modification of the protein in order to strengthen existing crystal contacts. The second step then consists of the further stabilization by cross-linking.
In my project, I am trying to perform such a modifications and achieve stable crystals of protein xylanase from the thermophilic fungus Thermoascus aurantiacus.
Author: Piotr Tokarz
Molecular Biotechnology, Jagiellonian University, Kraków
HZB-Summerstudent 2017


Very interesting project and well introduced. But the text is a bit too long. The image is super, it shows clearly the idea. I think you could have been a bit shorter on the introduction and maybe use a metaphor as a shortcut. Because – maybe I misunderstand? – to me it seems you are trying to invent a sort of scaffolding structure for proteins, is it? I would like to know more about it, you made me curious.
I am sure that your abstract has more than 800 characters, but still it was easy to read. I believe that your topic about macromolecular crystals should be presented during the final talk.
Up!
I like Proteins!
http://gph.is/1V6X9C8
Can you tell us more?