Let’s spray and create samples !
In the institute of Solar Fuel, the main research is to produce hydrogen from sunlight and water. Hydrogen can be used to easily store energy and also directly for transportation. But how can we produce it? Only from the sunlight, a metal oxide semiconductor and water. Photons from sunlight will be absorbed by the metal oxide, and this will generate electrons and holes which oxidize or reduce water. The reaction between the electron and hole and the electrolyte allows to separate water in oxygen and hydrogen.
Let’s see how we make this metal Oxide!
SPRAY PYROLYSIS:

The chemical method of spray pyrolysis is usually used in the institute of Solar Fuel. With this method, we spray a precursor solution on a pre-heated glass. This is how we prepare metal oxide semiconductor thin films from liquid phase. The used glass has a thin film of FTO (Fluorine doped Tin Oxide) on one side, which is optically transparent and electrically conductive. We deposit the thin films on FTO side, in order to have a conductive back contact to transfer the electrons.
HOW DOES IT WORK?
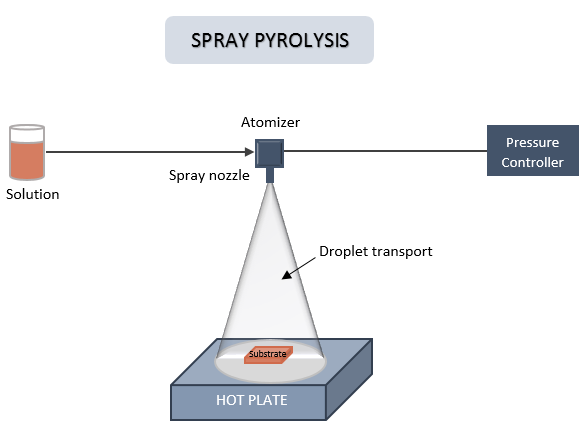
The FTO is placed on a hot plate throughout 10 minutes. Once the glass reaches the desirable temperature, we start to spray. During the whole process, the hot plate has a constant temperature of about 450°C.
The solution which is sprayed is a metal oxide. For instance, we use a combination of Bismuth and vanadium for depositing BiVO4. This is the semiconductor which will absorb the sunlight and can generate electrons and holes.
This solution is positioned at the same height as the atomizer. This atomizer is equipped with two controllers that allows to open the spray nozzle.
Furthermore, the installation is fitted with three valves to control the pressure, which the main one is an overpressure of nitrogen gas which come from outside. This control allows the user to set the amount of solution for a spray, i.e. the spray rate.
The spray isn’t continuous. This means, there are breaks between each spray. Spray’s length and the interval between two sprays is controlled by the user through a computer.

WHY THIS METHOD?

There are several deposition methods, such as sputtering, pulse laser deposition (PLD), atomic laser deposition (ALD), and spray pyrolysis, that allow us to deposit the metal oxide semiconductor on the substrate (glass coated with FTO).
So we can ask ourselves how to choose the right method?
The choice of the appropriate method depends on the material and the desirable characteristics. As an example, let’s take the case of BiVO4. We use spray pyrolysis because in the precursor solution, many organics (such as Ethanol, Acetic Acid and Bismuth Acetate) are present. The goal is to get only BiVO4 on the substrate, so we want to deposit only BiVO4 on the FTO and not the organic materials. The spray pyrolysis enables us to do this. Indeed, during the deposition, the FTO substrate is already heated and the organics do not resist heat. When the droplet of the solution reaches the FTO glass, the organics evaporate quickly. Thus, the goal is achieved.
Moreover, the film obtained by spray pyrolysis is porous and not dense. Other methods which are physical method such as PLD and sputtering, enable us to have a dense film.
However, it is possible to correct this density problem. After the spray application, we can heat the substrate to a high temperature of 460°C. In this way, the film becomes denser and we obtain a better crystallization of the metal oxide semiconductor on the FTO.
WHAT ABOUT THE LIMITS?

Since 2013, spray pyrolysis is used in the institute of Solar Fuel. It was set up by Dr. Fatwa F. Abdi. This technique was revolutionary for BiVO4 deposit. It’s a low-cost technique and it allows to spray homogeneous films which can be prepared in large-scale.
However, there are also some limits. There will always be instability due to uncontrollable external factors.
As it’s specify higher up, spray nozzle is driven by an overpressure of nitrogen gas which come from outside and sometimes the spray rate is decreasing during the deposition. It can be due to other experiments which are done in the institute (e.g. if people use the nitrogen in other labs at the same time, we experience lower pressure while depositing).
In my summer school program, I mainly aim to find out the reproducibility of the samples encountering this issue.
ATASAY Yasemin

Thank you for letting us know about this fine method!
Excellent blog !
I will spray organic semiconductors on conductive substrate. And this blog provides me ideas.