“If, in some cataclysm, all of scientific knowledge were to be destroyed, and only one sentence passed on to the next generations of creatures, what statement would contain the most information in the fewest words? I believe it is the atomic hypothesis (or the atomic fact, or whatever you wish to call it) that all things are made of atoms—little particles that move around in perpetual motion, attracting each other when they are a little distance apart, but repelling upon being squeezed into one another. In that one sentence, you will see, there is an enormous amount of information about the world, if just a little imagination and thinking are applied.”
Richard Feynman, The Feynman Lectures on Physics
Even when it was already well known that the matter consists of atoms, it was still impossible to identify the chemical composition of samples. Some kind of revolution was done after German chemist Robert Bunsen invented a special burner that is now called after his name. The main innovation was in fact that the flame produced by a Bunsen burner gave clean and colourless flame. That is why when you entered a sample inside you could see the changing of flame colour distinctly. For instance, the introduction of a strontium salt grain gave a bright raspberry fire. Calcium – brick red; barium – green; sodium is bright yellow.
Thanks to this discovery, it was possible to determine what does the substance consists of by the colour of the flame. But still there was a problem when a substance of several elements was introduced into the Bunsen burner flame, it was difficult to determine each element: one colour clogged another.
This problem was solved with the help of experienced physicist, Bunsen’s college – Gustav Kirchhoff. In 1859 he suggested looking not directly at the flame, but at its spectrum. The assembled Kirchhoff’s spectroscope according to the Fraunhofer principle made it possible to watch the hot steam of each element producing rays of a strictly defined colour that the prism deflected at the same angle to the same point on the screen.
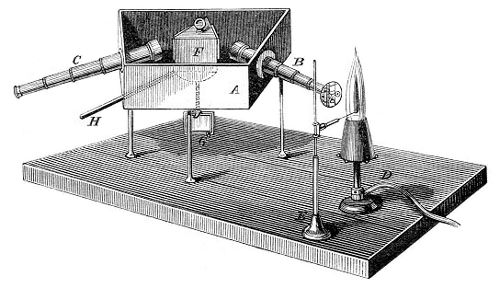
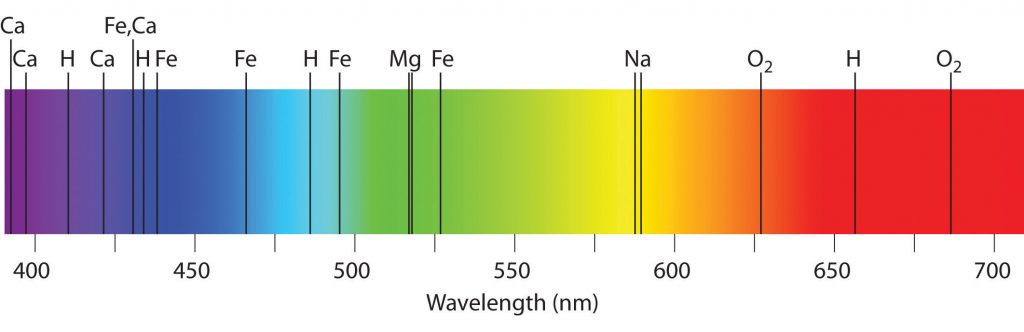
By sketching the spectrum of several individual elements Kirchhoff was able then to determine very accurately the composition of more complex substances. When they were added to the burner all the bright lines shone separately each on a certain position. The sensitivity of the spectrometer was so high that it was possible to determine the presence of an element weighing less than a milligram. Thus, Bunsen and Kirchhoff laid the foundations of spectral analysis, which after their work was introduced into the practice of chemical research.
The atomic spectroscopy invention was the revolution of the 19th century. The next one happened in 1944 in Kazan, where the new spectroscopic technique called Electron paramagnetic resonance (EPR) was introduced by physicist Yevgeny Zavoisky.
EPR is a spectroscopic technique that detects species that have unpaired electrons. It offers alone incontrovertible evidence of their presence. Actually, a surprisingly large number of materials have unpaired electrons: these include free radicals, many transition metal ions, and defects in materials. As a result, EPR crosses several disciplines including: chemistry, physics, biology, materials science, medical science and many more. Samples for EPR are very sensitive to local environments. So, the technique sheds light on the molecular structure near the unpaired electron. In addition, EPR has the unique power to identify the paramagnetic species that is detected.
One of the key areas where EPR spectroscopy is used is the correlation study between the structure and the magnetic properties of high-spin molecules and complex metal compounds. Nowadays, scientists all over the world develop molecular systems with high magnetic anisotropy, in other words, they create magnets that are comparable with the size of atoms. Such systems are commonly called single-molecule magnets (SMMs). They gained a huge interest, because SMMs are considered to have a great potential for new technological applications, for example in such areas as quantum calculations, the development of next-generation devices for recording and storing information, creation of multifunctional materials.
Moreover, the progress does not stand still and for new synthesized SMMs we need more efficient experimental spectroscopic techniques to investigate. There is one in HZB and it is called THz EPR which is an infrared spectrometer with additional magnet up to 10 T which uses the synchrotron facility as the radiation source. It allows you to determine certain magnetic parameters directly and opens up a wide range of possibilities for SMMs research.

You can find this setup in BESSY II synchrotron located on THz beamline. Come to visit it and measure new substances in order to learn a piece of “an enormous amount of information about the world”.

Great work. I love to read about the history of science and find it very helpful to introduce such concepts. Thanks a lot for this nice contribution!