An international team of scientists has greatly improved the stability of organic-inorganic lead halide perovskites. These materials have enormous potential for photovoltaic applications, but still suffer from relatively short device lifetimes.
Guanidinium replaces some traditional cations
The scientists, led by researchers from the EPFL in Lausanne, Switzerland, incorporated a large organic cation – guanidinium – into the perovskite crystal structure, partly replacing the methylammonium and formamidinium cations used traditionally.
Conversion efficiency 19 %
Overall, the new material delivered average power conversion efficiencies of over 19%, and increased operation to 1,000 h under continuous illumination. This is an important step for research in the perovskite field.
Scientists at HZB and HU
Two of the authors, Norbert Koch and Maryline Ralaiarisoa, are members of the Molecular Systems research group. This is a collaboration between the Helmholtz-Zentrum Berlin and the Humboldt-Universität zu Berlin. Maryline is working on her PhD and is enrolled in the HZB-HU hybrid4energy Graduate School.
Their results have been published in Nature Energy: Large guanidinium cation mixed with methylammonium in lead iodide perovskites for 19% efficient solar cells
Nature Energy 2, 972–979 (2017)
doi:10.1038/s41560-017-0054-3
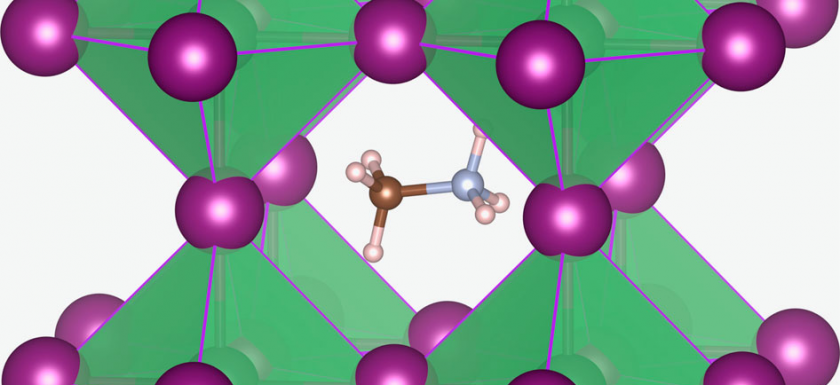
PS: The picture shown above this text is not given in the mentioned paper, but taken from Wikipedia, CC BY 4.0. It illustrates the “classical” organic-inorganic perovskite: A Methylammonium cation (CH3NH3+) is surrounded by 12 nearest-neighbour iodide ions in corner-sharing PbI6 octahedra. Credit: By Christopher Eames et al. – http://www.nature.com/ncomms/2015/150624/ncomms8497/full/ncomms8497.html, CC BY 4.0
