
“Be a diamond. Flourish under pressure” – Maureen Joyce Connolly
To most people diamonds are well-known as the marvelous gemstones which used in a piece of jewelry. However, they are not only “a girl’s best friends”. Due to their remarkable physical and optical properties diamonds can be used by scientists and engineers as well! With this blogpost I’ll guide you through my research here at HZB and, hopefully, will share the delight and enjoyment of working with diamonds.
Why diamonds?
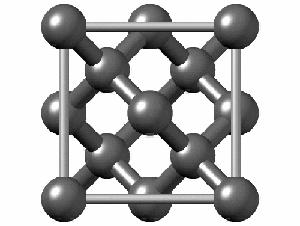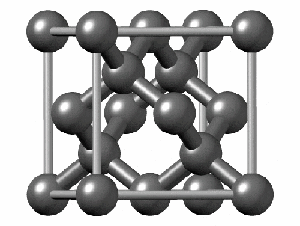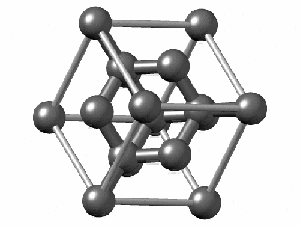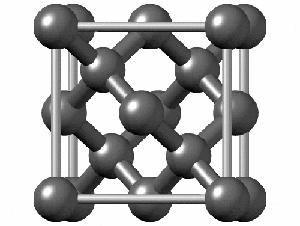
To have a better understanding of diamond it is useful to have a look at its crystalline structure. The strong sp3 covalent bonds between carbon atoms makes the lattice extremely rigid, forming one of the hardest materials with a value of 10 on the Mohs scale. Pretty impressive, right?
But this is not even the tip of the iceberg. To find even more outstanding properties one has to look at the optical features of a diamond. The transparency together with the high refractive index gives diamond its special sparking look. With this, if polished appropriately, the carbon modification becomes a valuable gemstone.

Since growing a pure diamond crystal is not an easy task to do, the lattice can incorporate certain impurities. In nature, those are primarily nitrogen and boron atoms, as they have roughly the same size as carbon atoms. The fancy thing is, the impurities can turn your transparent gem into beautifully coloured one.
And this is the point where the real magic happens.
Mysterious Spin
It has been known for decades that elementary particles have their own basic characteristics which describe the properties of a certain particle type. Indeed, we refer to them as “mass”, “charge” and “spin”. The first two attributes are perceived effortlessly cool, but spin is more of a weirdo. According to the definition given by Wikipedia, spin is an intrinsic form of angular momentum carried by elementary particles, composite particles, and atomic nuclei. The spin vector is the only quantity characterizing the orientation of a particle in QM. And the question we ask: is it possible to detect a remote nuclear spin?
Thanks to our shiny friends, we have an audacity to say yes!
Optically Detected Magnetic Resonance…
Let’s look at a bit unusual type of defects which is a nitrogen-vacancy centre. It consists of a substitutional N atom with an adjacent vacancy. This combination can be easily implanted within the diamond lattice. As an illustration, the NV-centre can be described as three level system with two triplet states and one effective intermediate state.
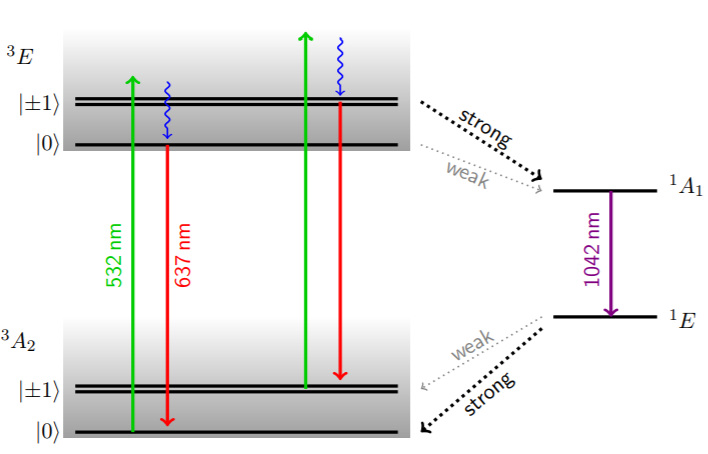
The NV-centre can be efficiently excited off-resonantly by laser illumination. Transitions between the 3A2 and 3E states are spin-conserving, but there is a second decay path over the intermediate singlets. Through these, the spin state can be changed.
The spin dependent fluorescence of the NV-centre together with the ability to drive the transitions with microwave radiation allows us to perform ODMR spectra. If the applied microwave frequency hits one of the allowed transitions, excitation of the spin state results in a drop of the recorded fluorescence.
… and More
Lastly, we apply external magnetic field parallel to the NV axis. As a result, one can see the Zeeman splitting on the spectra with a splitting value proportional to the magnetic field. In other words, any systems with the non-zero internal magnetic field distribution can be detected and carefully measured.
So, I guess, by now some of you are already overwhelmed by this amount of physics… But don’t worry, I still have a little yet lovely inside for you 🙂
To get a glimpse of a workday at HZB as a Summer Student, I have a short video, which I’d like to share with all of you today!
Thank you for watching!

Very interesting writing!
Congratulations for such an amazing skills to communicate your work.
Cheers from a 2017 summer student
I have viewed all the posts, but this one is out of competition. Brilliant work! And a special like for your video!
Such a girl
Makes spin twirl!
It can be guessed,
She is the best!
You gave tremendous positive points there. I did a search on the topic and found most peoples will agree with your blog.
Great work! Very intresting!