
Nanoparticles are tiny, really tiny. Only a fraction of a micrometer or some nanometers in size. And only some thousands of molecules strong, their properties can differ dramatically from those of their larger cousins. Titaniumdioxide, a whitish powder, can form such nanoparticles. In water, these nanoparticles can act as catalysts, when excited with light. and facilitate water splitting or water remediation.
However, what happens exactly when TiO2 and water get into contact and which impact this is having on the electronic structure of TiO2 remained in the dark: it is very tricky to probe nanoparticle–water interface experimentally and to observe changes.
Soft X-rays at BESSY II
One method is available at BESSY II: It is Soft X-ray absorption spectroscopy, which has already been used to characterize the electronic structure of TiO2 materials, but so far only in vacuum conditions.
Now, Freigeist-fellow Tristan Petit and his team could present a first study of TiO2 nanoparticles measured directly in aqueous dispersion. For this purpose, they developed a new method to probe nanomaterials in liquid using a holey membrane-based flow cell. With this approach, the X-ray transmission of the membrane was increased, especially in the water window, compared to solid membranes.
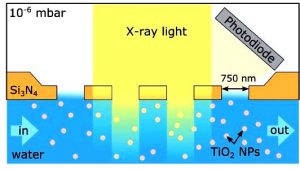
In their summary, they point out how Ti-O bonds can be distorted by interaction with water molecules. “These interactions may stabilize the smallest TiO2 nanoparticles, which have an electronic signature similar to amorphous films when dried in vacuum,” the scientists conclude.

More information:
The study was published in Advanced Materials Interfaces, (23/2017), december 2017. “Colloidal Systems: X-Ray Absorption Spectroscopy of TiO2 Nanoparticles in Water Using a Holey Membrane-Based Flow Cell”
