
Sunlight is a huge source of energy. However, it is not available 24/7. But, solar energy can be used to split water. Water splitting is the decomposition of water into oxygen and hydrogen gas due to the passage of an electric current induced by two electrodes in a conductive solution. This hydrogen can be stored or transported and be used later. Scientists at the Institute for Solar Fuels work on a way to split water efficiently by studing different type of electrodes. Usually, a high photocurrent during water splitting means a high production of hydrogen.
When I arrived for my internship at HZB in Wannsee, my supervisor, Dr. Ronen Gottesman, told me that I will be working on a device called the Scanning Droplet Cell (SDC) which can measure the photocurrent at different points of an electrode.
How does it work?
In fact, this device can measure electrodes with a gradient of thickness. Thanks to it, we can link the increase or the decrease of the photocurrent to the thickness of the material on the surface of the electrode. In my case, I have to measure the photocurrent of CuBi2O4 electrodes with a certain range of potential applied at every point.

The tip of the device moves on the surface of the electrode according to the coordinates I set up previously on the software. Nothing seemed really frightening at this point.
Why would it be scary to use the SDC?
When I started to acquaint myself with the device, I realised that my supervisor was the only one regularly using it. Other scientists who tried to use it find the software difficult to use, slow and capricious. They also warned me that the instrument by itself is very fragile and a lot of its components are expensive.
I started then to slow down my acquaintance with the device. I feared to break something, change the parameters by mistake or even lose previous data. And as the SDC measures photocurrent, the surface of the electrode is degraded during the experiment. Another reason for me to be extra careful.
My experience with the SDC
It took me nearly two weeks and two times reading the entire manual to begin my experiments. In between, I had numerous stressful moments where the software wasn’t responding, and I didn’t know what to do.
Then, I tried for the first time to do a measurement on a single point of my electrode. But no current occurred. I thought that I had break something. I had to contact my supervisor who was away at the time. It wasn’t easy to figure things out but finally I found out that one of the wire wasn’t correctly connected. Nothing serious, but I was so focused on messing up something important such as breaking the device that I couldn’t see my very little mistake. It took me nearly the all afternoon that day to figure it out.
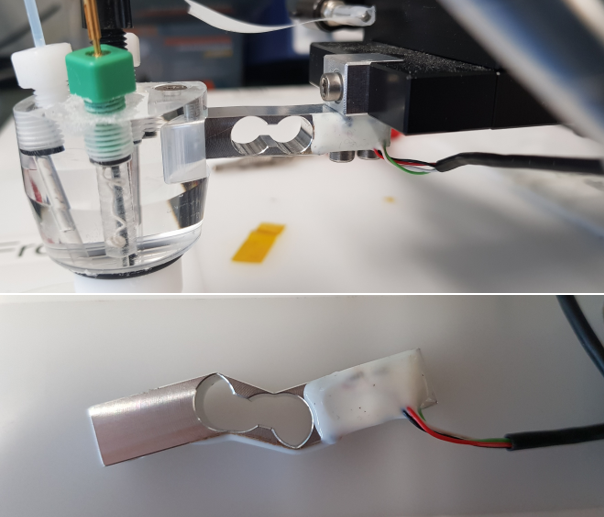
Another time, I also broke the force sensor. I don’t really know how, and I didn’t even notice that it was completely distorted.
Conclusion
After all, everything is well. I know nearly all the things I need to conduct a measurement. And I am not scared anymore to use the SDC.
We shouldn’t be afraid of learning about a device or a measurement. We all must learn at the beginning and most of the time, those devices are not perfect. As always with science, it was only a matter of time, patience, carefulness and about learning of my mistakes.

Thank you for sharing this. It is well written, sincere and concrete. Perseverance and patience are the virtues of true scientists….